Oxidation
Harness the Power of Oxygen in Organic Synthesis
Key Applications in Organic Chemistry
Oxidation reactions are fundamental in nature and essential in organic synthesis. In the chemical industry, they employ various oxidizers—ranging from molecular oxygen and hydrogen peroxide to osmium tetroxide. Using traceless gaseous reagents enables greener processes by reducing purification waste, since any excess gas can be easily removed. Oxidation reactions are important in organic synthesis, as they introduce or modify functional groups in molecules. However, autooxidations and other reactions with radical intermediates frequently exhibit low chemo- and regioselectivity. Stoichiometric metal oxidants, such as KMnO4 or K2CrO4 generate large quantities of waste.
Sustainable Solutions for Synthesis Challenges
The use of molecular oxygen minimizes chemical waste, producing water as the sole final by-product. The direct oxidation of organic substrates by O2 is rare, as the energy barrier for electron transfer from the organic substrate to the oxidant is usually high. For molecular oxygen, which has a triplet ground state, this high energy barrier is the way of nature to protect organic compounds from destructive oxidation. One of the disadvantages of using molecular oxygen is the difficulty to protect the starting material from over-oxidation. Accurate residence-time control in flow chemistry allows selective oxidation using inexpensive oxidizing agents. The H-Cube® Pro or the Phoenix Flow Reactor — equipped with the Gas Module — is capable of managing heterogeneously catalyzed oxidation reactions. It operates at pressures up to 100 bar and oxygen flow rates up to 100 mL/min.
Instruments for Catalytic Oxidations
H-Cube® Advance >>
New generation flow reactor, built for heterogeneous catalytic reactions. Enables applications at temperatures between 0-150°C and pressures up to 100 bar. Oxygen can be connected via the external gas inlet.
Phoenix Flow Reactor >>
High-performance flow reactor with an extended parameter window (temperature up to 450°C, pressure up to 200 bar), enabling the synthesis of novel compounds.
Catalytic Oxidation with the Phoenix Flow Reactor
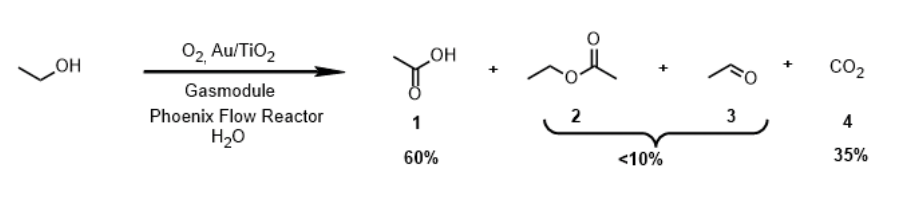
A ThalesNano study demonstrates how the Phoenix Flow Reactor combined with the Gas Module enables the efficient catalytic oxidation of ethanol to acetic acid under high-temperature, high-pressure gas–liquid–solid flow conditions. Using a gold-on-titania catalyst, the flow system achieved higher selectivity (up to 65%) and reduced by-product formation compared to traditional batch methods. It also maintained high ethanol conversion (over 90 %) at optimal temperatures. The setup also showed stable performance over extended operation, highlighting its potential as an environmentally friendly and scalable alternative to industrial acetic acid production. The work, carried out in collaboration with ETH Zürich, underscores the Phoenix Flow Reactor’s capability to open new chemical process windows beyond standard batch chemistry.
Do you have more questions?
Reach out to us at any time and experience fast and efficient support tailored to your specific needs.